NEWS
● Bioland obtains China FDA hygiene permit for collagen membrane (Product name: T-Gen) for dental use
● Company ready to explore Chinese market for surgical wound dressing (Product name: Colladerm)
● Explores new markets in Germany, Israel, Iran, etc. and plans to enter USA market
Bioland (CEO Jeong Chan-bok), Korea’s No. 1 supplier of cosmetics raw materials, has secured a bridgehead for entering the medical supplies market in China after more than three years of preparations. BioLand also disclosed that it has recently obtained a hygiene permit from the China Food and Drug Administration (CFDA) for its T-Gen collagen membrane for dental application. Acquisition of the hygiene permit, an essential step in promoting its medical supplies business in China, means the company has completed 80% of the path toward full-scale promotion of its business in China. Bioland has been supplying dental and surgical collagen membrane products in the local market ever since it entered the collagen-_ base_d medical supplies market in 2006, and has contributed to localization of the related products, which previously were heavily dependent on imports. It has also secured a _ base_ for advancing into foreign markets by obtaining the hygiene permit from CFDA.
Bioland targets Chinese dental supplies market - expected to grow rapidly
Dental collagen membrane is an essential medical material for implant surgery as it assists recovery after an implant procedure. Supply and demand of the product is affected by the scale and growth trend of the implant market, its main demand source. As the Chinese implant market was valued at around KRW 143 billion at end of 2015, and is growing at an annual average rate of 18.9%, it is expected that the demand for dental collagen membrane will also grow rapidly. BioLand plans to increase its share of the Chinese market for dental medical supplies through aggressive marketing toward Osstem Implant Inc., the No. 1 implant material supplier in China, and other global and local businesses in China.
Bioland’s surgical wound dressing also expected to obtain CFDA permit
It appears that Bioland’s surgical collagen medical supply business in China will achieve positive results sooner or later as its surgical collagen medical supply product, Colladerm, is currently under review and expected to obtain the CFDA hygiene permit in the first half of this year. Bioland was awarded a contract by Wuxi Huawei Pharmaceutical, a Chinese medical equipment supplier, to supply 30 billion won of wound dressings in 2014. The company will be able to actively explore the Chinese market for surgical wound dressing products in China once the CFDA procedure is successfully completed. “We plan to utilize China as our _ base_ for exploring the global collagen-_ base_d medical supplies markets,” announced Bioland CEO Jeong Chan-bok “We aim to become the global market leader by further enhancing our technology and quality on the basis of our ongoing R&D efforts.”
Company also exploring global dental supplies markets including Europe, Middle East and Americas
Bioland recently advanced into the European and Middle Eastern markets thanks to its efforts to explore new markets for its dental collagen membrane around the world, and is preparing to advance into the North American region in due course. It has also increased its product sales by signing supply contracts with local vendors in Germany, Israel, and Iran among other countries, and is currently in the process of obtaining a US FDA permit to enter the US market. [End]
Photos attached
1. Bioland logo

2. Product photos
a. Dental collagen membrane (product name: T-Gen)
b. Surgical collagen membrane (product name: Colladerm)

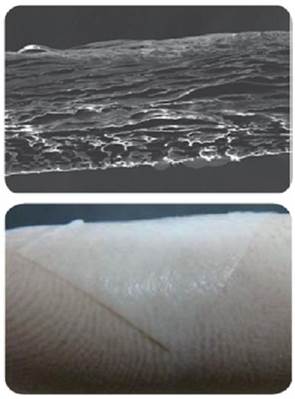
c. Microscopic photo of collagen membrane